Current and Emerging Treatment
Early-stage AMD treatments
While far more common, no approved treatment exists for early age-related macular degeneration (AMD), and management options consist of reduction of risk factors and nutritional supplements, as described in the previous section. Evaluating approaches to managing early to intermediate AMD, particularly atrophic AMD, is currently a focus of research.1–4
Patients may be given an Amsler grid to self-evaluate weekly for any changes in vision, such as dark spots, missing areas of the grid, or distorted/wavy lines. See below for examples of potential visual changes that may be reported while using an Amsler grid for self-evaluation, which could indicate fluid accumulation inside or behind the retina.5
Providers also can ask patients to test their vision in each eye by regularly covering one eye at a time and reading, noting any new vision changes. Follow-up should be arranged at individualized intervals, with the understanding that patients should return promptly if they experience any new symptoms.
Treatment of geographic atrophy (advanced atrophic, non-exudative, or “dry” AMD)
A part of the immune system called the “complement cascade” has long been identified as a culprit in AMD. Recently approved therapies targeting elements of the complement cascade, pegcetacoplan (C3, OAKS, and DERBY) and avacincaptad pegol (C5, GATHER 1/2), are now available as injectables, aimed at stopping immune attacks on the retina that result in geographic atrophy. While they have been shown to slow the development of geographic atrophy by up to 20%, they do not improve vision.1,4,6
Emerging treatments for atrophic AMD
Several innovative treatments for atrophic AMD are in progress; some studies have used medication to target the inflammation associated with AMD, while others have looked at therapies that reduce the effects of oxidative stress. Researchers also have considered stem cell-based therapies to regenerate damaged photoreceptors and retinal pigment epithelium (RPE).4,7
In 2023, two intravitreal therapies were approved for non-neovascular AMD with GA. Typically injected on a monthly or bimonthly basis, they act to slow GA progression.
- Pegcetacoplan 15 mg/0.1 mL blocks the C3 and C3b proteins of the complement-mediated immune system.
- Avacincaptad pegol intravitreal solution 2 mg is a PEGylated, stabilized aptamer that targets C5.3
Treatment of choroidal neovascularization (CNV); advanced neovascular, exudative, or “wet” AMD
Neovascular AMD (nAMD) treatment has seen significant advancements in recent years. Given the central role of vascular endothelial growth factor (VEGF) in nAMD pathogenesis, anti-VEGF intravitreal agents are recommended for first-line treatment and limit the destructive effects of neovascularization on retinal tissue. Anti-VEGF agents bind VEGF in the eye, including within the retina, preventing activation of VEGF receptors on retinal blood vessels. By blocking VEGF receptor activation, vessel leakiness and abnormal vessel growth can be decreased, as well as cause new vessel growth that was stimulated by excess VEGF to recede.8,9
These injections can stabilize or possibly reverse vision loss. Therapy should be initiated at the first signs and symptoms of neovascular disease, as smaller lesions respond better to treatment. Any delay in therapy (more than 21 weeks) is associated with poorer vision outcomes.2-4
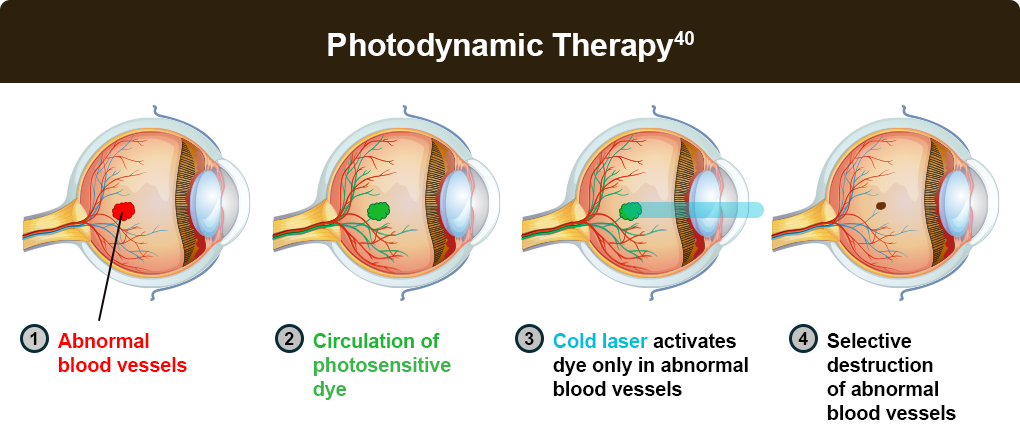
Photodynamic therapy and laser photocoagulation are other treatment options for those who do not respond to anti-VEGF therapy.
First-generation anti-VEGF therapy
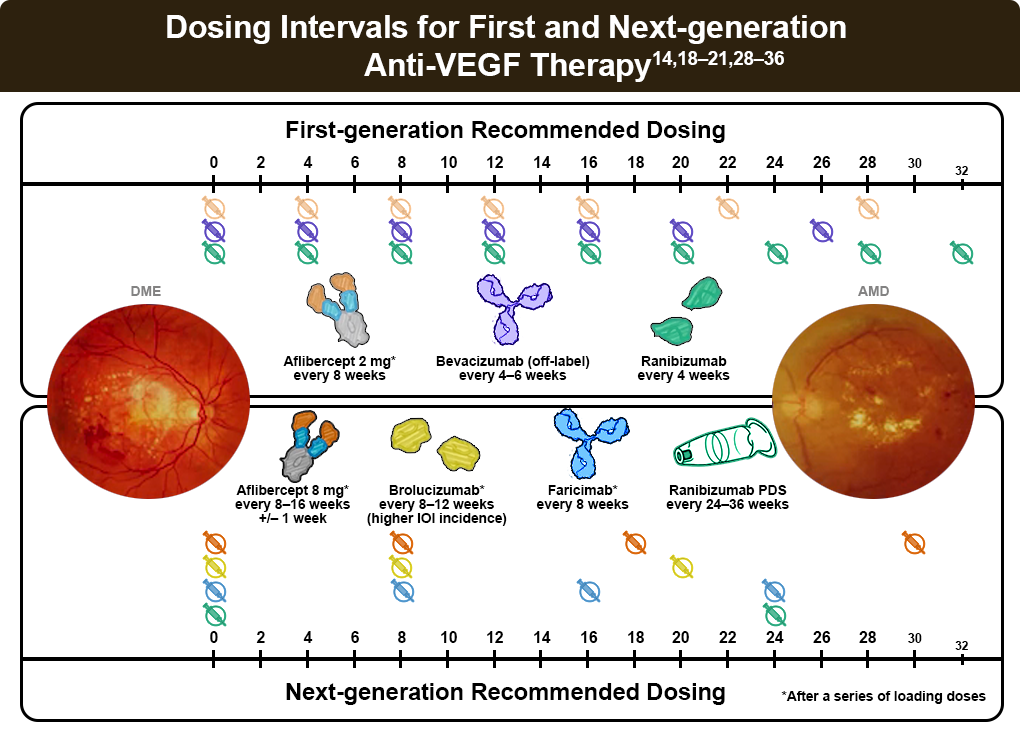
Currently available first-generation VEGF inhibitors delivered by intravitreal injection include aflibercept (2 mg), repackaged bevacizumab, and ranibizumab. Generally well tolerated, the most common adverse reactions include conjunctival hemorrhage, eye pain, vitreous floaters, and increased intraocular pressure.1,3,4 Reviews of numerous randomized trials suggest the systemic safety and efficacy profiles of bevacizumab, ranibizumab, and aflibercept appear similar.10,11
- Aflibercept (2 mg) targets all VEGF-A isoforms and placental growth factor (PIGF). It has a recommended dosing schedule of every 4-8 weeks.12
- Repackaged bevacizumab, originally approved as a treatment for colon cancer, has been used to treat nAMD “off-label” due to effectiveness, cost, safety, and insurance requirements. The recommended dosing schedule is every 4–8 weeks. Concerns for a greater possibility of infection with bevacizumab exist due to potential contamination when the drug is repackaged into smaller doses; however, risk is minimized by following appropriate guidelines for preparation.12
- Ranibizumab is a humanized monoclonal antibody fragment targeting the receptor-binding site of VEGF-A, with a recommended dosing schedule of every 4–8 weeks.12
- Biosimilar agents for aflibercept (2 mg) and ranibizumab have also been approved by the US Food and Drug Administration (FDA), with studies on bevacizumab currently underway. Biosimilars are highly similar to FDA-approved biological medicines, demonstrating no clinically meaningful difference and noninferior efficacy to the original.13
Dosing regimens
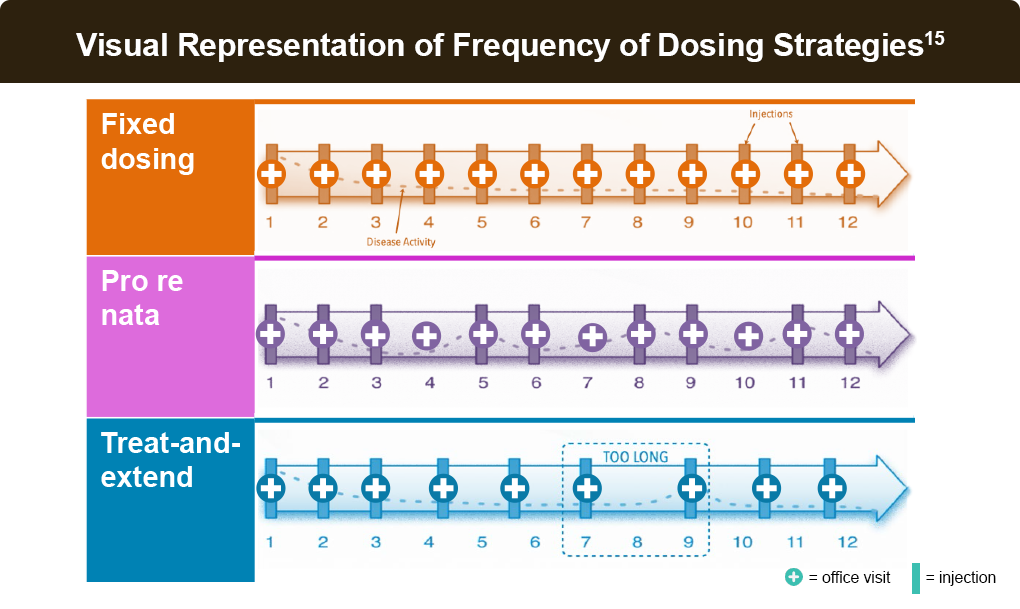
Alternative anti-VEGF dosing protocols include pro re nata (PRN) or treat-and-extend. These protocols are initiated with an induction phase – usually 3 months – of fixed monthly dosing.14
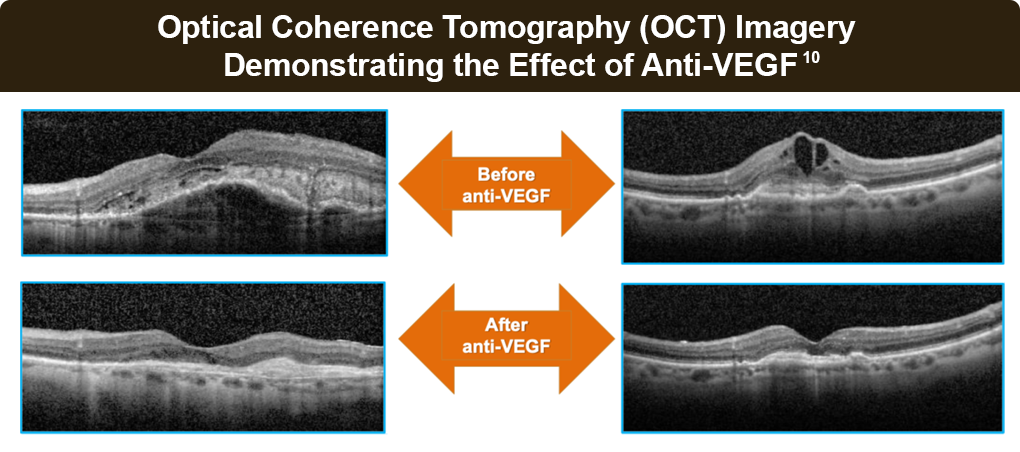
PRN (as needed) dosing involves monthly evaluations during which injections are either given or withheld based on (OCT) findings of active exudation or stable disease, respectively. PRN regimens require frequent monitoring visits to sustain visual acuity gains, which may be impractical in a real-world setting.14
The treat-and-extend strategy involves intravitreal injection at each visit, even if no disease activity is observed. However, if macular fluid is found to be suppressed or eliminated on OCT, the duration to the next visit is extended by a preplanned increment. Conversely, if fluid recrudescence is found, an injection is made, and the interval to the next visit is shortened. The treat-and-extend approach offers better visual and anatomical outcomes than PRN while easing treatment burden in real-world settings.14
Regardless of approach, the durability of first-generation agents impacts injection interval, and the burden of frequent injections may impact long-term patient adherence.8 Various social factors can also contribute to challenges with consistent follow-up, leading to missed appointments, irregular treatment, and ultimately, negative vision outcomes.16
To maintain the benefits of anti-VEGF treatments, consistent, frequent follow-up and retreatment are necessary. Although dosing strategies aim to mitigate treatment burden–while still leading to acceptable vision outcomes–they fail to sustain initial vision gains for many patients.17
Next-generation anti-VEGF therapy
Newer anti-VEGF agents include sustained-release formulations, hardware delivery systems, and therapies with longer durations of effect that allow for longer treatment intervals, which may reduce burden and improve treatment adherence.14,18
- Aflibercept 8 mg was designed with a 4-times-higher molar dose to provide longer effective vitreal anti-VEGF concentration. The PULSAR trial demonstrated safety and efficacy as compared to 2 mg every 8 weeks.19–21 Extended dosing intervals of aflibercept 8 mg, up to 16 weeks in the first year and potentially up to 24 weeks in the second year, could decrease the burden of treatment without increases in observed immunogenicity.11-13
- Faricimab, a dual-target monoclonal antibody that targets both VEGF-A and angiopoietin-2, has demonstrated non-inferiority to existing anti-VEGF treatments.14 About half the patients in the TENAYA and LUCERNE phase 3 trials for nAMD were able to defer injection to every 16 weeks, suggesting that longer injection intervals did not compromise the quality of care.14
- Brolucizumab, targeting 3 VEGF-A isoforms with a concentrated molar dose, has also shown efficacy with more than 50% of patients moving to 12-week dosing intervals through the HAWK and HARRIER trials. These trials linked brolucizumab to a higher incidence (4%) of intraocular inflammation, retinal vasculitis, and retinal vascular occlusion, prompting risk-benefit assessment for patient selection and close treatment monitoring.22–25 Brolucizumab is approved for use every 8 to 12 weeks following the loading period, and more frequent administration should be avoided.3
- Ranibizumab, delivered via a port delivery system implanted in the sclera, continuously delivers VEGF-A targeting through passive diffusion and is refilled every 6 months. The Archway phase 3 trial demonstrated non-inferiority to monthly intravitreal ranibizumab.26–28
Individualizing therapy
The role of VEGF and the inflammatory response is not the same for every patient, and patients can exhibit a range of responses to therapy. A case-by-case approach that considers factors relating to each patient’s clinical features, needs, and preferences drives the choice of which agent or agents to use and how often to use them.37
Should a patient experience persistent fluid, new fluid accumulation, or desire increased treatment intervals between injections, clinicians can consider switching to an alternate anti-VEGF agent.38
When considering treatment options for nAMD, clinicians should consider disease severity, potential adherence challenges, cost, treatment-associated risks, and patient preference.17,39
Photodynamic and laser therapy
Emerging therapies for nAMD
While existing therapies for nAMD primarily target VEGF-A, other VEGF targets are being considered. Sozinibercept (OPT-302) is a novel trap fusion protein that inhibits VEGF-C and VEGF-D, ligand mediators of angiogenesis and vascular leakage involved in retinal vascular diseases. VEGF-C/D signal for angiogenesis and vascular permeability independently of VEGF-A and are elevated when VEGF-A is inhibited. Used in combination with a VEGF-A inhibitor, sozinibercept completely blocks VEGFR-2 and VEGFR-3 signaling, broadly suppressing VEGF/VEGFR pathways, which improve clinical efficacy and durability.41
Gene therapy is also a promising treatment for nAMD to inhibit or change the pathogenesis of the disease. ABBV-RGX-314 is an adeno-associated virus serotype 8 vector that expresses an anti-VEGF-A antigen-binding fragment, which provides potential for continuous VEGF-A suppression after a single subretinal injection. In the AAVIATE clinical trial, subretinal delivery of ABBV-RGX-314 was generally well tolerated with no clinically recognized immune responses. ABBV-RGX-314 gene therapy provides a novel approach for sustained VEGF-A suppression in patients with nAMD that has potential to control exudation, maintain vision, and reduce treatment burden after a single administration.42
References
- Fernandes AR, Zielińska A, Sanchez-Lopez E, et al. Exudative versus nonexudative age-related macular degeneration: Physiopathology and treatment options. Int J Mol Sci. 2022;23:2592.
- Cunningham J. Recognizing age-related macular degeneration in primary care. JAAPA. 2017;30:18-22.
- Vemulakonda GA, Bailey ST, Kim SJ, et al. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology. 2025;132(4):P1-P74.
- Flores R, Carneiro Â, Vieira M, Tenreiro S, Seabra MC. Age-related macular degeneration: Pathophysiology, management, and future perspectives. Ophthalmologica. 2021;244:495-511.
- Larsen S. Low Vision Specialists. Home Testing With The Macular Degeneration Grid Test. https://lowvisionaids.org/blog/macular-degeneration-grid-test-do-at-your-home/
- Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: A randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128:576-586.
- Khurana RN, Garg SJ. New Treatments for Age-Related Macular Degeneration. American Academy of Ophthalmology. May 23, 2024. https://www.aao.org/eye-health/tips-prevention/promising-new-treatments-amd
- Mehta H, Tufail A, Daien V, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127-146.
- Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012;87:77-88.
- Optical coherence tomography image attribution: Dr. Carl Regillo
- Kaiser PK, Singer M, Tolentino M, et al. Long-term safety and visual outcome of intravitreal aflibercept in neovascular age-related macular degeneration. Ophthalmol Retina. 2017;1:304-313.
- Mukamel R. Comparison of Anti-VEGF Treatments for Wet AMD. American Academy of Ophthalmology. May 23, 2024. https://www.aao.org/eye-health/diseases/avastin-eylea-lucentis-difference
- Hariprasad SM, Gale RP, Weng CY, Ebbers HC, Rezk MF, Tadayoni R. An introduction to biosimilars for the treatment of retinal diseases: A narrative review. Ophthalmol Ther. 2022;11:959-982.
- Almony A. Treatment approaches for neovascular age-related macular degeneration and diabetic macular edema. Am J Manag Care. 2023;29(6 suppl):S81-S89.
- Lanzetta P, Loewenstein A; Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: Optimal application of intravitreal anti–vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255:1259-1273.
- Skelly A, Bezlyak V, Liew G, Kap E, Sagkriotis A. Treat and extend treatment interval patterns with anti-VEGF therapy in nAMD patients. Vision (Basel). 2019;3:41.
- Weng CY, Singh RP, Gillies MC, Regillo CD. Optimizing visual outcomes in patients with neovascular age-related macular degeneration: The potential value of sustained anti-VEGF therapy. Ophthalmic Surg Lasers Imaging Retina. 2023;54:654-659.
- Ben-Arzi A, Ehrlich R, Neumann R. Retinal diseases: The next frontier in pharmacodelivery. Pharmaceutics. 2022;14:904.
- Kaiser SM, Arepalli S, Ehlers JP. Current and future anti-VEGF agents for neovascular age-related macular degeneration. J Exp Pharmacol. 2021;13:905-912.
- Lanzetta P, Korobelnik JF, Heier JS, et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet. 2024;403:1141-1152.
- Wykoff CC, Brown DM, Reed K, et al. Effect of high-dose intravitreal aflibercept, 8 mg, in patients with neovascular age-related macular degeneration: The phase 2 CANDELA randomized clinical trial. JAMA Ophthalmol. 2023;141:834-842.
- Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72-84.
- Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: Ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128:89-99.
- Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion–related events with brolucizumab: Post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128:1050-1059.
- Tadayoni R, Sararols L, Weissgerber G, Verma R, Clemens A, Holz FG. Brolucizumab: A newly developed anti-VEGF molecule for the treatment of neovascular age-related macular degeneration. Ophthalmologica. 2021;244:93-101.
- Xu M, Fan R, Fan X, Shao Y, Li X. Progress and challenges of anti-VEGF agents and their sustained-release strategies for retinal angiogenesis. Drug Des Devel Ther. 2022;16:3241-3262.
- Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: Results from the randomized phase 2 ladder clinical trial. Ophthalmology. 2019;126:1141-1154.
- Ranade SV, Wieland MR, Tam T, et al. The port delivery system with ranibizumab: A new paradigm for long-acting retinal drug delivery. Drug Deliv. 2022;29:1326-1334.
- Puliafito CA, Wykoff CC. Looking ahead in retinal disease management: Highlights of the 2019 angiogenesis, exudation and degeneration symposium. Int J Retina Vitreous. 2019;5:22.
- Chakravarthy U, Peto T. Current perspective on age-related macular degeneration. JAMA. 2020;324:794-795.
- Li G, Zhu N, Ji A. Comparative efficacy and safety of Faricimab and other anti-VEGF therapy for age-related macular degeneration and diabetic macular edema: A systematic review and meta-analysis of randomized clinical trials. Medicine (Baltimore). 2023;102:e36370.
- Khanna S, Komati R, Eichenbaum DA, Hariprasad I, Ciulla TA, Hariprasad SM. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: A comparative review. BMJ Open Ophthalmol. 2019;4:e000398.
- Brown DM, Boyer DS, Do DV, et al. Intravitreal aflibercept 8 mg in diabetic macular oedema (PHOTON): 48-week results from a randomised, double-masked, non-inferiority, phase 2/3 trial. Lancet. 2024;403:1153-1163.
- EYLEA® (aflibercept). Prescribing information. Regeneron Pharmaceuticals, Inc; 2023. https://www.regeneron.com/downloads/eylea_fpi.pdf
- Nanegrungsunk O, Au A, Sarraf D, Sadda SR. New frontiers of retinal therapeutic intervention: A critical analysis of novel approaches. Ann Med. 2022;54:1067-1080.
- Lucentis® (ranibizumab). Prescribing information. Genentech, Inc; 2024. https://www.gene.com/download/pdf/lucentis_prescribing.pdf
- Wykoff CC, Clark WL, Nielsen JS, Brill JV, Greene LS, Heggen CL. Optimizing anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration. J Manag Care Spec Pharm. 2018;24(2-a suppl):S3-S15.
- Jaffe GJ, Kaiser PK, Thompson D, et al. Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology. 2016;123:1856-1864.
- Maturi RK, Glassman AR, Josic K, et al. Effect of Intravitreous anti–vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: The Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139:701.
- Photodynamic Therapy. Macula Retina Vitreous Center. https://macularetinavitreouscenter.com/services/surgeries-procedures/photodynamic-therapy/
- Opthea highlights latest phase 3 trials for sozinibercept. Ophthalmology Times. April 12, 2024. https://www.ophthalmologytimes.com/view/opthea-highlights-latest-phase-3-trials-for-sozinibercept
- Campochiaro PA, Avery R, Brown DM, et al. Gene therapy for neovascular age-related macular degeneration by subretinal delivery of RGX-314: A phase 1/2a dose-escalation study. Lancet. 2024;403:1563-1573.
All URLs accessed May 25, 2025